Resources
Terms of use
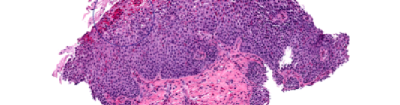
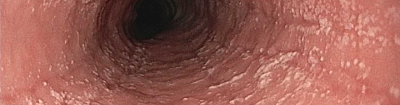
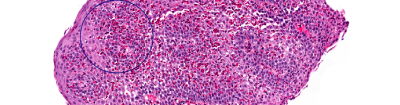
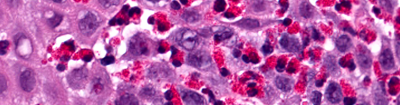
Thank you for your interest in participating in EGID Partners, a patient-centered research study. "Patient-centered research" means that patients and researchers partner with each other and work as a team. Your input is critical to the research process. Working together, patients and scientists hope to improve the quality of life for patients with EGIDs.
By joining EGID Partners you can contribute to EGID research in many ways, such as by completing surveys, providing additional information about your health, and submitting research questions. You will team up with researchers to answer important questions about EGIDs.
Who is sponsoring this study?
This research study was designed by researchers on the steering committee at University of North Carolina, Wake Forest University, Vanderbilt, and patient advocacy groups including CURED (Campaign Urging Research for Eosinophilic Disease), APFED (American Partnership For Eosinophilic Disorders), and EFC (Eosinophilic Family Coalition). In addition, some funding for EGID Partners has been provided by Shire, which is now a subsidiary of Takeda. This means that the research team is being paid by the sponsors for doing the study. Data collected in this study include treatment information for specific diseases, including the use of drugs that are manufactured by a variety of pharmaceutical companies. However, you data will always be secure, as noted in our privacy policy. If you would like more information, please send an email to info@egidpartners.org
Here’s what you can expect:
If you choose to participate, we will ask you to provide information about your disease and treatment by completing online surveys every 6 months. If you do not feel comfortable answering a survey question, you do not have to answer it.
We will touch base with you periodically to let you know about the progress of the program and to provide information about EGIDs. You can log on to our website to view up-to-date information about the study. Every 6 months, we will ask you to provide an update about your treatments and health. From time to time, we will let you know about additional surveys and research studies for which you might be eligible.
All data that you enter will be stored in a secure manner and may be retained by EGID Partners indefinitely, even after you terminate your account. No personal information will be released to an outside party or disclosed publicly; however, de-identified health data may be used for research purposes. This includes studies that may be funded by non-profit or for-profit entities.
Here’s what we’ll do:
Your privacy matters to us. We will work diligently to protect the health data we collect from you during this study. The EGID Partners research team will never release your contact information to anyone at any time without your permission. Identifiers, such as your name and address, will not be used in research and will be maintained securely. Any research study associated with new data collection must first undergo review and approval by a EGID Partners Steering Committee. Counts of patients who meet certain criteria are sometimes needed to assist in planning studies. At times we will make this aggregate count data available to qualified researchers prior to committee review.
We will never sell your data to drug companies for market research. Qualified researchers will be able to explore the data in EGID Partners for insights into the causes of and potential treatments for EGID. These qualified researchers come from academic, government, non-profit, and for-profit organizations (including pharmaceutical companies) that in some instances contribute funds to support its mission. We will also notify you about research findings published in abstracts and scientific papers that used EGID Partners data.
Here’s what you need to know as you get started:
Completing the confidential baseline survey should only take about 30 minutes of your time. The survey is implemented by the EGID Partners Data Management Center at the University of North Carolina at Chapel Hill (UNC). Your email address is linked to your survey so we can contact you in the future. Your information is kept completely confidential on UNC's secure servers. For details, please click here to see our Privacy policy and Data Security Measures.
Keep in mind all questions are optional; that is, you don't have to answer any questions that you don't want to answer. You can withdraw from the study at any time. We will not associate you with any response in any reports of survey results. There is no cost to you. You may stop participating at any time by contacting the Research Team at info_egidpartners@unc.edu.
Again thank you for helping us. We believe this project has excellent long-term potential to improve our understanding of EGIDs and the quality of life of patients with EGIDs